University Study Finds Cannabis Compound Inhibits SARS-CoV-2 in Human Lung Cells
A study from the University of Chicago in Illinois has shown yet another incredible application for the cannabis plant. Researchers discovered in the study that a particular cannabis compound inhibits infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human lung cells.
Marsha Rosner and her colleagues from the university fount cannabidiol (CBD) and its metabolite 7-OH-CBD potently blocked SARS-CoV-2 replication in lung epithelial cells.
“CBD could be acting to block viral entry to host cells or at later steps following infection. As CBD was shown to decrease ACE2 expression in some epithelial cells including A549, we first determined whether CBD suppressed the SARS-CoV-2 receptor in our A549-ACE2 overexpressing cells,” Rosner and the authors wrote.
According to the study, researchers assessed the incidence of SARS-CoV-2 infection among 82 patients who had been prescribed CBD prior to SARS-C0V-2 testing and matched patients who had not been prescribed CBD. What they found was astounding.
The study found the incidence of SARS-CoV-2 was only 1.2% among the patients prescribed CBD, compared with 12.2% among the matched patients who had not been taking CBD.
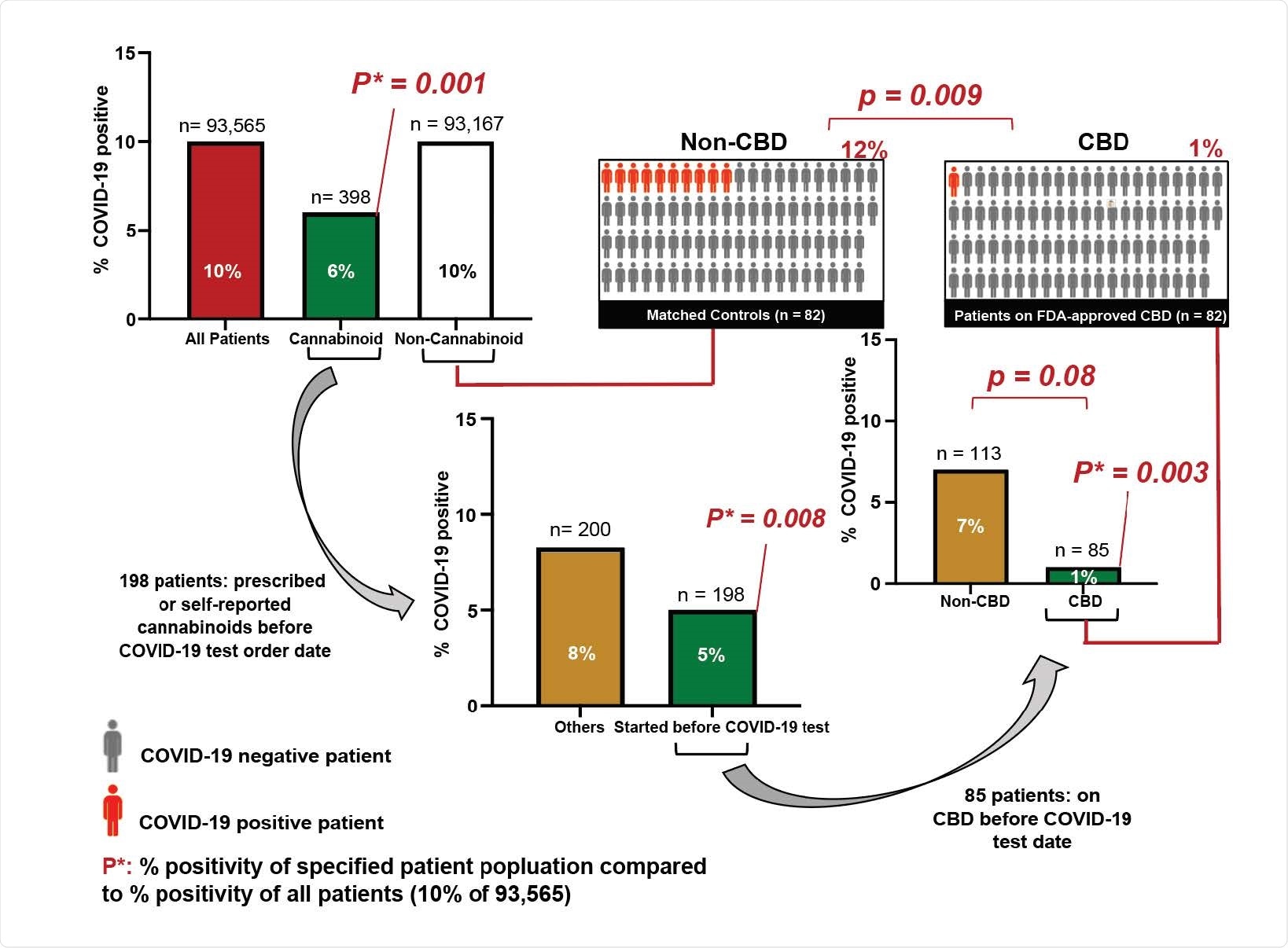
The substantial reduction in SARS-CoV-2 infection risk of approximately an order of magnitude in patients who took FDA-approved CBD highlights the potential efficacy of this drug in combating SARS-CoV2 infection.
We advocate carefully designed placebo-controlled clinical trials with known concentrations and highly-characterized formulations in order to define CBD’s role in preventing and treating early SARS-CoV-2 infection.
It is important to point out that this study is currently in peer review, and, therefore, should not be regarded as conclusive.
Because CBD is currently approved in the US for use in all 50 states, this news, though only in its infant stages, is optimistic, to say the least.
As Journal Development Editor for BioMed Central (BMC) Sally Robertson points out,“although recently-approved vaccines are now being rolled out in many countries, the virus is still spreading rapidly. Rosner and colleagues say this highlights the need for alternative approaches, particularly among populations with limited access to vaccines.”
The SARS-CoV-2 virus primarily enters host cells through the binding of a surface viral protein called spike to the human host cell receptor angiotensin-converting enzyme 2 (ACE2).
The viral genome is then translated into two large polypeptides that are cleaved by the viral proteases MPro and PLPro to produce the proteins required for viral replication, assembly, and budding.
Rosner and colleagues say that, although limited, some studies have reported that certain cannabinoids have antiviral effects against hepatitis C virus and other viruses.
According to the study, the same metabolite that treats epilepsy — 7-OH-CBD — is likely the same one which block SARS-CoV-2 infection at early stages of infection and is associated with a lower risk of SARS-CoV-2 infection in humans. Researchers say that these results show promise that cannabis compounds could potentially fight other viral infections in the future.
The substantial reduction in SARS-CoV-2 infection risk of approximately an order of magnitude in patients who took FDA-approved CBD highlights the potential efficacy of this drug in combating SARS-CoV-2 infection. Finally, the ability of CBD to inhibit replication of MHV raises the possibility that CBD may have efficacy against new pathogenic viruses arising in the future.
Researchers point out that CBD as a treatment for COVID-19 is advantageous over other treatments as the negative side effects from ingesting the compound are almost non-existent in most humans.
CBD has a number of advantages as a potential preventative agent against SARS-CoV-2. CBD is widely available without restricted access if the content of THC is <0.3%. There are multiple means of ingestion, including potential for inhalation and nasal delivery. CBD blocks viral replication after entry into cells and, thus, is likely to be effective against viral variants with mutant spike proteins. Unlike drugs such as remdesivir or antiviral antibodies, CBD administration does not require injection in hospital settings. Finally, CBD is associated with only minor side effects.
The study made the important distinction, however, that not all CBD is created equal and formulations on the market vary widely in quality. They also pointed out that THC may act to counter the CBD antiviral efficacy, eliminating the possible feasibility of marijuana as a similar treatment.
Researchers conclude that further research for delivery methods for optimal CBD uptake is need to fully test the promise of CBD as a therapeutic to block SARS-CoV-2 infection.
Future studies to explore the optimal means of CBD delivery to patients along with clinical trials will be needed to fully test the promise of CBD as a therapeutic to block SARS-CoV-2 infection. As the clearance rates for CBD in plasma are substantially lower in humans than mice, we would suggest moving to clinical trials rather than doing preclinical studies in animal models. We advocate carefully designed placebo-controlled clinical trials with known concentrations and highly-characterized formulations in order to define CBD’s role in preventing and treating early SARS-CoV-2 infection. The necessary human in vivoconcentration and optimal route and formulation remain to be defined. We strongly caution against the urge to take CBD in presently available formulations as a preventative or treatment therapy at this time, especially without the knowledge of a rigorous randomized clinical trial with this natural product.
This is the second such study which ties cannabis to the potential reduction of SARS-CoV-2 infection on which TFTP has reported. In the other study, out of Canada, researchers found that certain strains of cannabis may also increase resistance to the coronavirus.
Spread the love




 thefreethoughtproject.com
thefreethoughtproject.com







